発熱物質検査市場は、予測期間中に約12.5%の成長率を記録すると予想されています

発熱物質検査市場は、予測期間中に約12.5%の成長率を記録すると予想されています。このレポートは、製品、テストの種類、アプリケーション、および地域によってセグメント化されています。市場は主に、製薬およびバイオテクノロジー業界における発熱性試験製品の需要の増加と、研究開発への投資の増加によって牽引されています。十分に発達した医療インフラの存在、および医薬品製造プロセスに関する明確に定義された規則と規制は、北米が予測期間中に市場シェアを支配するのに役立つはずです
慢性疾患の罹患率は先進国で高く、新興市場では高い速度で成長しています。したがって、発熱物質検査の需要と意識の高まりは、予測期間中に高い成長をもたらすと予想されます
この市場を牽引するその他の要因は、ライフサイエンス業界における研究開発への多額の投資、製薬およびバイオテクノロジー企業の数の増加、生物学的製剤の発売の増加、および新しい革新的な医薬品の急増です
主な市場動向
単球活性化試験は、最も高い増殖速度を目撃することが期待されています
発熱物質の測定は、非経口投与の医薬品および医療機器にとって不可欠な安全対策です。単球活性化試験(MAT)は、ヒト単球または単球細胞を活性化してヒト発熱応答に関与する内因性メディエーターを放出する物質を検出または定量するために使用されます。ウサギ発熱物質試験の代替案は、発熱物質誘導サイトカインの発熱誘導機構を考慮して開発された。単球活性化試験(MAT)は、例えば、発熱物質によってインビトロで刺激されたヒト血液単球または単球細胞株において誘導されるIL−6またはIL−1bの検出に基づいている。ブラジル薬学ジャーナルによって、単球活性化試験が製薬業界でより注目を集めており、それが同種の急速な成長に貢献していると示唆されています
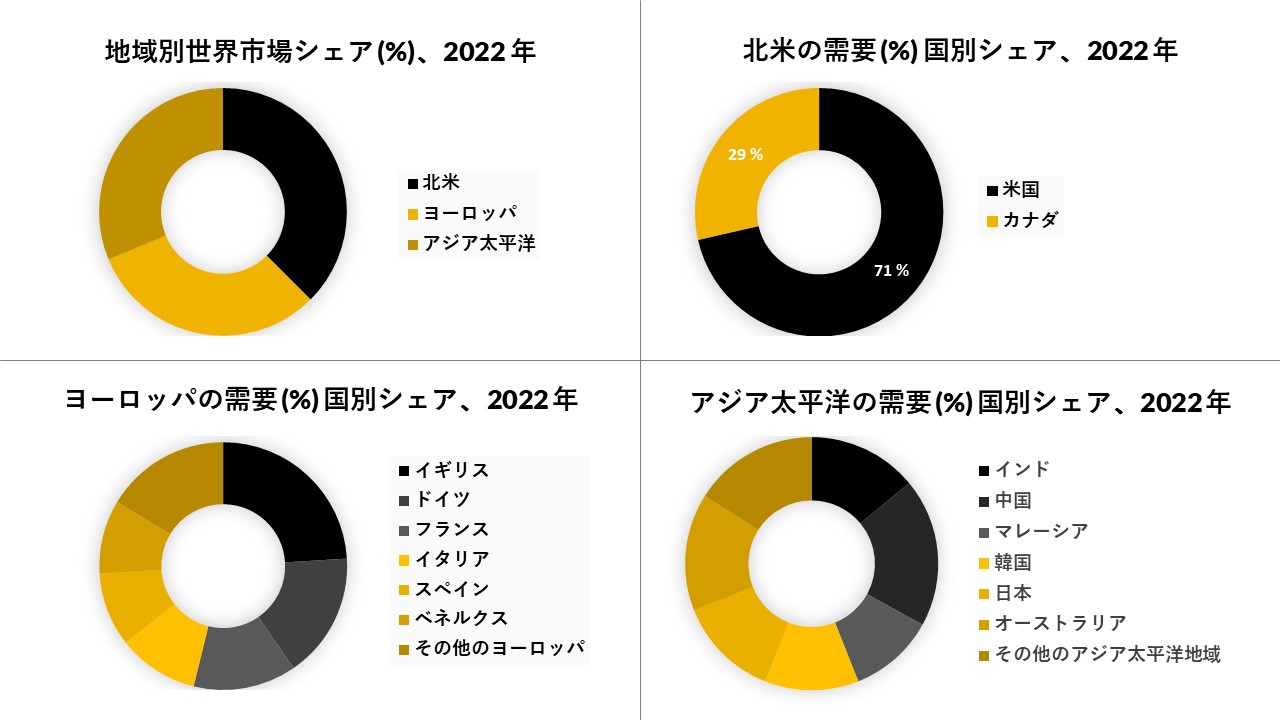
北米は予測期間
で市場を支配すると予想されます
北米は、確立された医療インフラ、より良い規制枠組み、メルク、ロンザグループなどの多くの大手バイオテクノロジーおよびバイオ医薬品企業の政府支援とプレゼンスにより、市場シェアを支配すると予想されています。これらの企業は、いくつかの政府機関や投資家からの資金提供の形で政府からの継続的な支援を受けて、新薬や生物製剤の開発に重点を置いており、この市場の成長を促進しています。アジア太平洋地域は最も高い成長率を示すと予想されています。インド、中国、日本などの国々での医療費の増加により、健全なCAGRが期待されています
競争環境
発熱物質検査の市場は適度に競争が激しく、メルクKGaA、ロンザグループ、バイオメリュー、ジェンスクリプトなどのいくつかの主要企業によって支配されています。のイノベーションの高まりは、市場に参入し、市場シェアを獲得するために競争を投げかけている斬新なプレーヤーの参入を促進しました.
このレポートを購入する理由:
- エクセル形式の市場予測(ME)シート
- 3ヶ月のアナリストサポート

北米(米国およびカナダ)、ラテンアメリカ(ブラジル、メキシコ、アルゼンチン、その他のラテンアメリカ)、ヨーロッパ(英国、ドイツ、フランス、イタリア、スペイン、ハンガリー、ベルギー、オランダおよびルクセンブルグ、NORDIC(フィンランド、スウェーデン、ノルウェー) 、デンマーク)、アイルランド、スイス、オーストリア、ポーランド、トルコ、ロシア、その他のヨーロッパ)、ポーランド、トルコ、ロシア、その他のヨーロッパ)、アジア太平洋(中国、インド、日本、韓国、シンガポール、インドネシア、マレーシア) 、オーストラリア、ニュージーランド、その他のアジア太平洋地域)、中東およびアフリカ(イスラエル、GCC(サウジアラビア、UAE、バーレーン、クウェート、カタール、オマーン)、北アフリカ、南アフリカ、その他の中東およびアフリカ)