ラテラルフローアッセイ市場調査レポート、規模とシェア、成長機会、及び傾向洞察分析―アプリケーション別、製品タイプ別、技術別、エンドユーザー別、サンプルタイプ別、及び地域別―世界市場の見通しと予測 2026-2035年
出版日: Feb 2026

- 2020ー2024年
- 2026-2035年
- 必要に応じて日本語レポートが入手可能
ラテラルフローアッセイ市場規模
2026―2035年のラテラルフローアッセイ市場の市場規模はどれくらいですか?
ラテラルフローアッセイ市場に関する弊社の調査レポートによると、市場は予測期間2026―2035年において約6%の複利年間成長率(CAGR)で成長すると予想されています。来年には、市場規模は235億米ドルに達する見込みです。しかし、弊社の調査アナリストによると、基準年の市場規模は133.2億米ドルでしました。
市場シェアの観点から、ラテラルフローアッセイ市場を支配すると予想される地域はどれですか?
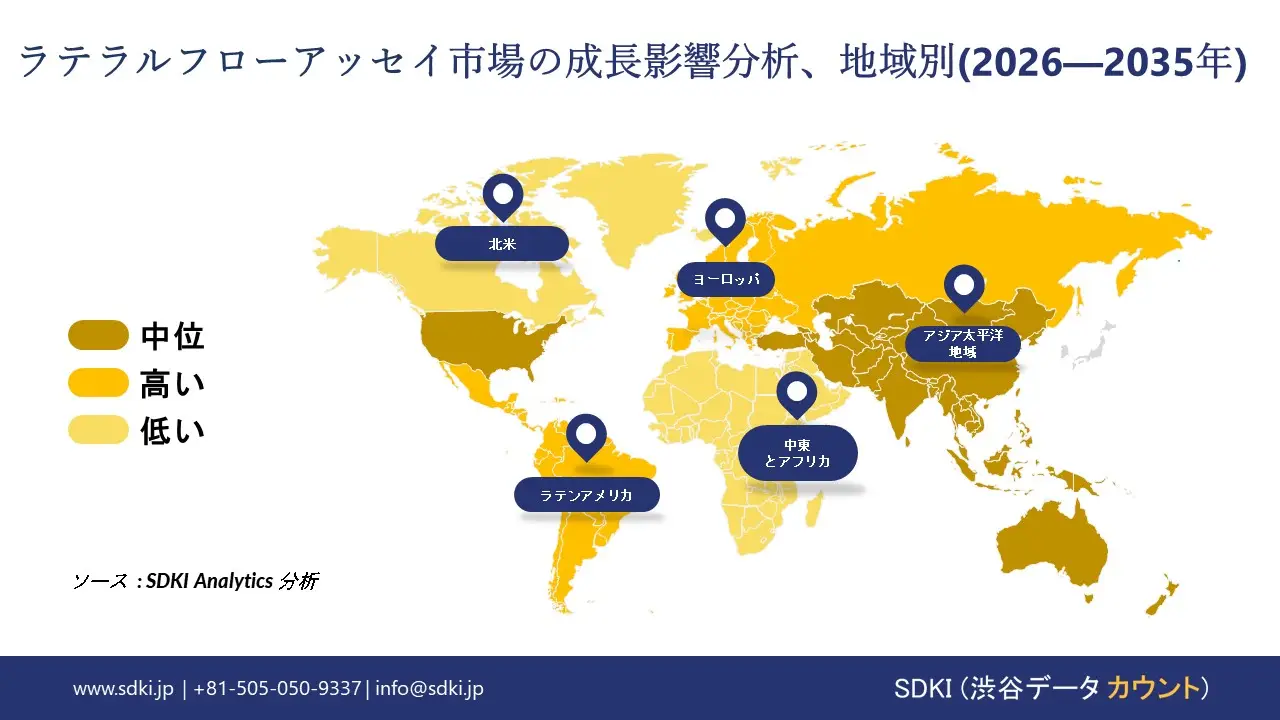
ラテラルフローアッセイに関する弊社の市場調査によると、北米市場は予測期間中、約35%の市場シェアを占め、市場を牽引すると予想しています。一方、アジア太平洋地域市場は今後数年間、有望な成長機会を示すと見込まれています。この成長は、主にポイントオブケア診断の需要の高まりと、政府の支援策によって推進されています。

ラテラルフローアッセイ市場分析
ラテラルフローアッセイとは何ですか?
ラテラルフローアッセイは、複雑な実験設備を必要とせずに、液体サンプル中の特定物質の有無を検出する簡便な分析方法です。さらに、このアッセイは、サンプルを多孔質ストリップに沿って移動させることで機能します。この移動により、サンプルは埋め込まれた試薬と相互作用し、標的分子が存在すると可視信号を生成します。この設計により、多くの場合数分以内に結果を直接読み取ることができるため、スピード、使いやすさ、そして最小限のトレーニングが重要となる環境に適しています。
ラテラルフローアッセイ市場の最近の傾向は何ですか?
弊社のラテラルフローアッセイ市場分析調査レポートによると、以下の市場傾向と要因が市場成長の中核的な原動力として貢献すると予測されています。
- 公衆衛生システムがLFAのアクセスと調達チャネルを制度化します-
弊社の調査レポートによると、主要な医療システムが日常的なアクセス経路にLFAを組み込み、信頼性の高い資金による需要の底値を維持するという静かで永続的な変化により、市場の見通しは好ましいものになると予想されています。イングランドでは、NHSイングランドののラテラルフローデバイス(LFD)検査供給サービスは、約3.9百万人の患者が地域の薬局を通じて無料のLFDキットを受け取る資格があり、タイムリーな‑抗ウイルス治療の検証が必要な可能性のある高リスクの人々に、5つの検査キットがウォークインベースで提供されると推定しています。このサービスは継続的に全国的に提供されており、運用の詳細が指定されています。
供給面では、NHSサプライチェーンが正式にLFD調達(UKHSAより)を引き継ぎ、最低発注数量を100件に設定し、国家製品コードKBD85000と2‑日以内配送のSLAを発表しました。これは、LFDが緊急用品ではなく、標準的な消耗品として扱われるようになったことの証です。さらに、FDAのCLIAプログラムは、免除対象検体と市販薬検査に関する公開データベースを継続的に更新しており、分散型施設(小売店、救急診療所、診療所)へのLFAのローリング配布をサポートすることで、COVID-19の波が過ぎても、ポイントオブケアのスループットを維持しています。
- マラリアの永続的な負担により、迅速抗原LFAの需要が構造的に高く維持されていますー
弊社の調査レポートによると、市場見通しはマラリアの負担の増大によって好ましい影響を受けると予測されています。マラリアの診断は、コミュニティレベルでの治療を導くために、主にラテラルフロー免疫測定法である迅速診断テスト(RDT)に大きく依存しています。たとえば、世界保健機関の世界マラリア報告書では、マラリア症例は282百万件、死亡者は610,000人と推定されており、2023年と比較して約9百万件の増加となっています。WHOは、2022~2024年に拡大される中核的介入には、蚊帳、化学予防、ワクチンに加えてRDTが含まれることを強調しています。レポートの付録では、流行国全体にわたる物資の配布と適用範囲を追跡しており、‑国家プログラムを通じた大規模なRDTの流れを強調しています。
地域によってLFAの普及率は異なり、その実態も異なります。WHOアフリカ地域は症例数の約94%と死亡者の大半を占めており、プログラム的な検査の需要は本質的に高いです。一方、大メコン圏では、WHOは在来種の熱帯熱マラリア(熱帯熱マラリア原虫)症例が89%減少したことを記録しています。これは成功と言えるものの、再流行を防ぎ、混合種間の動態を管理するためには、RDTの継続的な使用が依然として必要です。このように、マラリア症例管理におけるRDTの規模、資金の可視性、そして運用上の重要性は、 ‑LFAに対する予測可能な複数年にわたる需要基盤を形成しています。
ラテラルフローアッセイ市場におけるラテラルフローアッセイの輸出に関して、日本の地元企業はどのような利益を得るのですか?
ラテラルフローアッセイ市場は、関税上の優遇措置や構造を考慮した輸出チェーンの価値向上という観点から、日本の市場プレーヤーにとって戦略的な機会を提供しています。ラテラルフローアッセイの実際の輸出額は日本税関貿易統計には記載されておらず、関税統計検索システムを用いたHSコード照会が必要となりますが、日本の輸出業者には消費税の免税措置があり、輸出価格設定における優位性を高めています。これにより、一定の基準を満たす物品の輸出は、適切な行政手続きを経て報告されれば、消費税が免税となります。
RCEPや二国間EPA(例えば日本とインド間のEPA)といった日本の経済連携協定(EPA)は、HSコードに記載されている原産地規則を満たす製品について、輸出業者に有利な関税削減条件を提供しています。輸出業者はEPAに基づき、関税の減免について有利な条件を得ることができ、輸出機会の拡大につながります。
公式の関税統計ツールキットではHSコードに基づいて輸出を詳細に分析できますが、ラテラルフローアッセイの実際の輸出数はすぐには分かりません。弊社の市場調査レポートによると、Sysmex Corporationをはじめとする日本の診断機器メーカーは、会社概要において海外市場と輸出国の成長を報告しており、輸出志向型であることを示唆しています。
ラテラルフローアッセイ市場に影響を与える主な制約は何ですか?
ラテラルフローアッセイは、PCRやELISAなどの高精度診断法と比較すると感度と特異度が低いため、懐疑的な見方をされることも少なくありません。全体的な性能が低いと、臨床医の信頼が低下し、規制当局が確認検査を要求する可能性があり、検査費用が上昇して市場導入が遅れる場合があります。日本とヨーロッパでは、適応症に対するさらなる制限や、非常に厳しい規制プロセスによる承認の遅れが生じる可能性があります。SDKI市場展望によると、特定のLFAタイプの感度は68%と低いことが実証されており、2023年の調査では、アジアの診断ラボの27%が、特にHIV及び希少疾患分野において、他の技術が好まれる主な理由の1つは感度不足であると考えています。
サンプル納品物ショーケース
- 調査競合他社と業界リーダー
- 過去のデータに基づく予測
- 会社の収益シェアモデル
- 地域市場分析
- 市場傾向分析
ラテラルフローアッセイ市場レポートの洞察
ラテラルフローアッセイ市場の今後の見通しはどのようなものですか?
SDKI Analyticsの専門家によると、ラテラルフローアッセイ市場の世界シェアに関連するレポートの洞察は以下の通りです。
|
レポートの洞察 |
|
|
2026-2035年の CAGR |
6% |
|
2025年の市場価値 |
133.2億米ドル |
|
2035年の市場価値 |
235億米ドル |
|
過去のデータ共有 |
過去5年間 2024年まで |
|
将来予測 |
2035年までの今後10年間 |
|
ページ数 |
200+ページ |
ソース: SDKI Analytics 専門家分析
ラテラルフローアッセイ市場はどのようにセグメント化されていますか?
ラテラルフローアッセイ市場の展望に関連する様々なセグメントにおける需要と機会を説明する調査を実施しました。市場はアプリケーション別、製品タイプ別、技術別、エンドユーザー別、サンプルタイプ別に分割されています。
ラテラルフローアッセイ市場はアプリケーション別によってどのように区分されていますか?
ラテラルフローアッセイ市場はアプリケーション別に基づいて、臨床診断、獣医診断、食品安全と環境試験、医薬品開発と品質試験に分割されています。 臨床診断の市場シェアは、感染症や慢性疾患の規制対象検査のポイントオブケア量が主にこのシェアを支え、さらに、構造上迅速で使い捨てのLFAを好む米国の医療システムで毎年行われる12億件以上の体外診断検査によってさらに支えられるため、2035年まで68%で推移すると推定されています。米国FDAの510(k)及びCLIA免除フレームワークは、約140の迅速抗原検査及び血清検査の承認に責任を負っており、償還可能な臨床展開を直接拡大するものであり、この市場が厳しく規制される一因となっています。Abbott Laboratoriesは、2024年のフォーム10-Kで、診断部門の収益は84億ドルを超え、ラテラルフロープラットフォームが利益率の高い迅速検査ポートフォリオを支えていると述べたように、企業の投資はこの市場の優位性を明確に示しています。経済的な観点から見ると、臨床LFAは検査あたりのコストが実験室での免疫測定よりも30%~50%低いため、2035年まで病院や診療所の注目を集めています。世界的に見ると、OECD諸国が全世界のIVD支出の75%以上を占めているという事実によって、臨床診断の需要は維持されるでします。
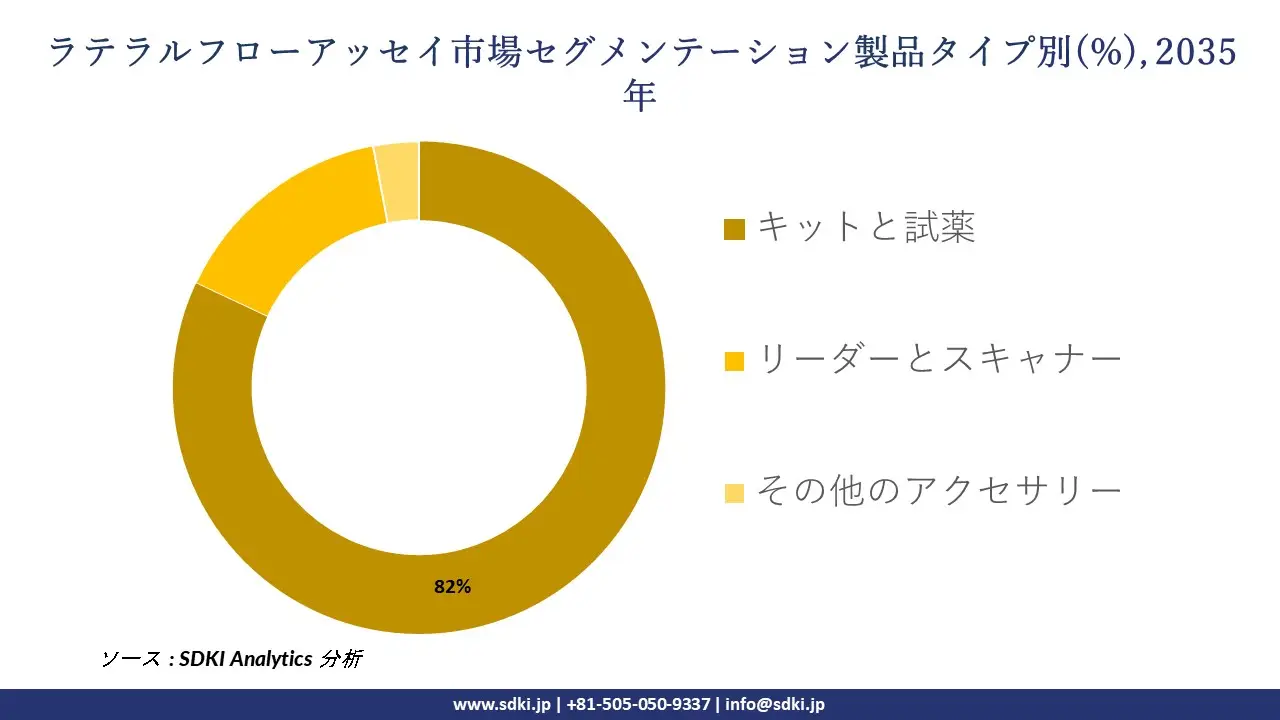
ラテラルフローアッセイ市場は製品タイプ別によってどのように区分されていますか?
さらに、ラテラルフローアッセイ市場は、製品タイプ別に基づいて、キットと試薬、リーダーとスキャナー、その他のアクセサリーに分割されています。 2035年までにキットと試薬の市場シェアは82%に達すると予測されています。これは、実施されるすべての検査には消耗品が不可欠であり、米国国勢調査局によると、2023年の免疫診断試薬の年間出荷額は190億米ドルを超えているため、消耗品の販売が徐々に標準になりつつあるためです。FDAのIVDラベルとロット出力が必須となり、各検査は検証済みの消耗品を使用して実施する必要があるため、規制当局をはじめとする関係者がこの傾向を牽引しています。そのため、標準キットの利用は臨床現場から在宅ケアユニットまで拡大しています。企業発表もこれを裏付けています。Roche Diagnosticsの2024年度年次報告書によると、消耗品の売上高は96億スイスフランを超えており、再検査経済により試薬が機器の売上を上回っています。技術面では、キットは大量調達において1検査当たりのコストを5米ドル未満に抑える可能性があり、読影検査に依存する方式よりも経済面で優れています。地域別に見ると、NMPA規制下にある中国の生産を代表とするアジア太平洋地域の生産規模は、世界の迅速検査消耗品の40%以上を供給し続けており、長年にわたり中国市場の優位性を確固たるものにしています。
以下は、ラテラルフローアッセイ市場に該当するセグメントのリストです。
|
親セグメント |
サブセグメント |
|
アプリケーション別 |
|
|
製品タイプ別 |
|
|
技術別 |
|
|
エンドユーザー別 |
|
|
サンプルタイプ別 |
|
ソース: SDKI Analytics 専門家分析
ラテラルフローアッセイ市場傾向分析と将来予測:地域市場展望概要
アジア太平洋地域のラテラルフローアッセイ市場は、9%を超える複利年間成長率(CAGR)で世界市場で最も高い成長率を記録すると予測されています。強力な結核検出プログラムが、この地域の市場成長を支えています。インドの報道情報局の報道によると、インドの国家結核撲滅プログラムでは、2024年にインドで26.07 ラックインドルピー件の結核症例が記録され、過去最高の報告数となりました。
結核と診断される人の急増は、監視体制の強化、検査へのアクセス拡大、そして公衆衛生に対する政府の取り組み強化を反映しています。こうした大規模な検出活動は、ラテラルフローアッセイなどの迅速診断ツールへの依存度の高まりを示しており、アジア太平洋地域はポイントオブケア検査ソリューションの拡大において重要な地域となっています。
SDKI Analyticsの専門家は、ラテラルフローアッセイ市場に関するこの調査レポートのために、以下の国と地域を調査しました。
|
地域 |
国 |
|
北米 |
|
|
ヨーロッパ |
|
|
アジア太平洋地域 |
|
|
ラテンアメリカ |
|
|
中東とアフリカ |
|
ソース: SDKI Analytics 専門家分析
北米のラテラルフローアッセイ市場の市場パフォーマンスはどうですか?
弊社のSDKI市場調査アナリストは、北米のラテラルフローアッセイ市場が予測期間を通じて35%以上の圧倒的な市場シェアを獲得し、世界市場で主導的な地位を確保すると予測しています。市場の成長は、規制当局の承認取得によるものです。
米国疾病予防管理センターの報告によると、2024年までに米国では、米国食品医薬品局によって一般使用が認可された市販のSARS-CoV-2迅速抗原検査製品が38タイプ存在していたことが判明しました。
この承認件数の急増は、強力な規制当局の支援を浮き彫りにし、信頼性の高い在宅診断の広範な普及を確かなものにしています。FDA承認済みの検査への消費者の容易なアクセスは、パンデミックへの備えを強化するだけでなく、ポイントオブケアソリューションへの信頼を高めることにもつながります。その結果、北米のラテラルフローアッセイ市場は、利便性、安全性、そして政府による検証を背景に、導入拡大の恩恵を受けています。
ラテラルフローアッセイ調査の場所
北米(米国およびカナダ)、ラテンアメリカ(ブラジル、メキシコ、アルゼンチン、その他のラテンアメリカ)、ヨーロッパ(英国、ドイツ、フランス、イタリア、スペイン、ハンガリー、ベルギー、オランダおよびルクセンブルグ、NORDIC(フィンランド、スウェーデン、ノルウェー) 、デンマーク)、アイルランド、スイス、オーストリア、ポーランド、トルコ、ロシア、その他のヨーロッパ)、ポーランド、トルコ、ロシア、その他のヨーロッパ)、アジア太平洋(中国、インド、日本、韓国、シンガポール、インドネシア、マレーシア) 、オーストラリア、ニュージーランド、その他のアジア太平洋地域)、中東およびアフリカ(イスラエル、GCC(サウジアラビア、UAE、バーレーン、クウェート、カタール、オマーン)、北アフリカ、南アフリカ、その他の中東およびアフリカ
競争力ランドスケープ
SDKI Analyticsの調査者によると、ラテラルフローアッセイの市場見通しは、大規模企業と中小規模企業といった様々な規模の企業間の市場競争により、細分化されています。調査レポートでは、市場プレーヤーは、製品や技術の投入、戦略的パートナーシップ、協業、買収、事業拡大など、あらゆる機会を捉え、市場全体の見通しにおいて競争優位性を獲得しようとしていると指摘されています。
ラテラルフローアッセイ市場で事業を展開している世界有数の企業はどれですか?
弊社の調査レポートによると、世界的なラテラルフローアッセイ市場の成長に重要な役割を果たしている主な主要企業には、 Abbott Laboratories、QuidelOrtho Corporation、Bio - Rad Laboratories、Inc.、Abingdon Health、Thermo Fisher Scientific、Inc.などが含まれます。
ラテラルフローアッセイ市場で競合している主要な日本企業はどこですか?
市場展望によると、日本のラテラルフローアッセイ市場のトップ5プレーヤーは、Nihon Shinobiological Co., Ltd.、Abcam, Adtec Co., Ltd.、 Kohjin Bio Co., Ltd.、Medical & Biological Laboratories Co., Ltd.などです。
市場調査レポート研究には、世界的なラテラルフローアッセイ市場分析調査レポートにおける主要プレーヤーの詳細な競合分析、企業プロファイル、最近の傾向、主要な市場戦略が含まれています。
ラテラルフローアッセイ市場における最新のニュースや傾向は何ですか?
- 2025年11月、Sapphiros はロシュと戦略的契約を締結し、10億個のラテラルフロー検査と将来の分子ポイント‑オブケア技術へのアクセスを獲得しました。この開発により、生産能力の拡大と広範な供給の確保が可能になり、世界のラテラルフローアッセイ市場が強化され、ラテラルフロー検査は世界中で迅速診断の基盤として位置付けられます。
- 2025年7月、Volitionは画期的なラテラルフロー検査を発表し‑、POC(ポイントオブケア)におけるヌクレオソーム定量を可能にしました。これにより、腫瘍学をはじめとする疾患の診断能力が向上します。この進歩は、感染症検査だけでなくがん診断にもアプリケーション範囲を拡大し、臨床現場におけるイノベーションと普及を促進することで、世界のラテラルフローアッセイ市場を支えます。
ラテラルフローアッセイ主な主要プレーヤー
主要な市場プレーヤーの分析
日本市場のトップ 5 プレーヤー

目次

ラテラルフローアッセイマーケットレポート
関連レポート
よくある質問

- 2020ー2024年
- 2026-2035年
- 必要に応じて日本語レポートが入手可能






