使い捨てデバイス(SUD)の再処理は、コストを節約し、医療廃棄物を削減するのに役立ちます

使い捨てデバイス(SUD)の再処理は、コストを節約し、医療廃棄物を削減するのに役立ちます。使い捨てデバイスの再利用には、規制上、倫理的、医学的、法的、経済的問題が含まれ、20年以上にわたって非常に物議を醸してきました。しかし、そのコスト削減の性質と持続可能性のために、医療機器の使い捨て再処理は現在、有望な見通しとして認識されつつあります。コスト削減とともに、環境の持続可能性にもつながります。SUDの再処理は、コストを削減し、リソースを最適化するために使用されるトップヘルスケアサプライチェーン戦略の1つです
主な市場動向
クラスIIデバイスセグメントは堅調な成長を記録する態勢が整っています
- デバイスタイプに基づいて、市場はクラスIとクラスIIのデバイスにセグメント化されています。クラスIIデバイスは、心臓介入の数の増加と、SUDの再利用を評価するための結果としての経済的負荷需要により、最高のCAGRを記録すると予想されています
- 米国心臓不整脈学会は、FDAに電気生理学的デバイスの再処理に好意的な意見を発表した。FDAは、電気生理学的心臓カテーテルをクラスII外科用装置に分類し、米国での再処理を許可しています
- しかし、過去数年間のこれらのデバイスのコストの全体的な削減と完全な消毒を保証することの難しさにより、それらを再利用する必要性が生じており、市場の成長を促進することが期待されています
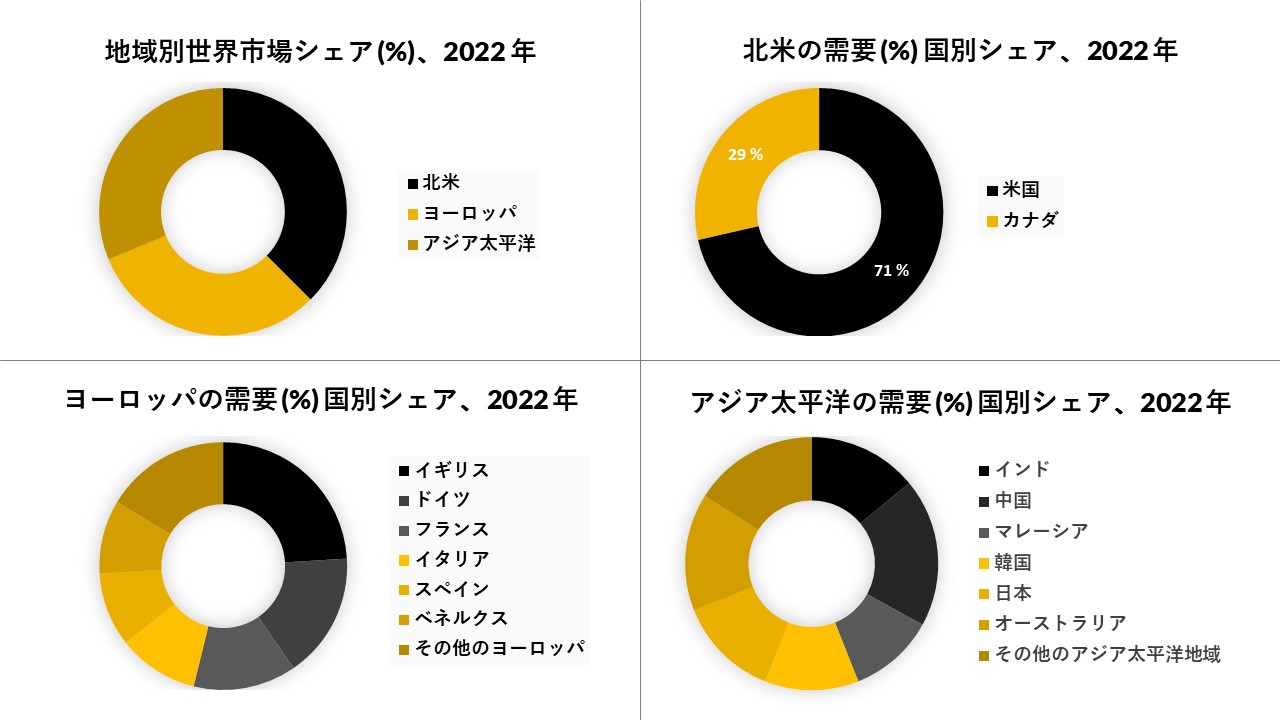
米国は使い捨て医療機器再処理市場を支配する
- 米国は最大の市場を占めました。米国に続いて、収益の面では、次の主要地域であるヨーロッパ諸国が続きます。さらに、アジア太平洋地域では使い捨て医療機器の再処理市場が成長しており、アジアのほとんどの地域で使い捨て医療機器の再利用が一般的になりつつあります
- しかし、ほとんどの場合、SUDの再利用を規制する国内規制がないため、サードパーティの再処理者はアジア太平洋地域の新興経済国のほとんどでサービスを提供していません
競争環境
世界的には、主要プレーヤーが調査対象の市場のかなりのシェアを支配しています。しかし、残りの市場シェアは大きく断片化しており、いくつかのニッチプレーヤーが地元市場で事業を展開しています。主要なグローバルプレーヤーには、SureTek Medical、Medline Industries Inc.、ReNu Medical(Arjo)、NEScientific Inc.、Sterilmed Inc.(Johnson & Johnson)、SteriPro、Stryker Corporation、Innovative Healthなどが含まれます><。
このレポートを購入する理由:
- エクセル形式の市場予測(ME)シート
- 3ヶ月のアナリストサポート

北米(米国およびカナダ)、ラテンアメリカ(ブラジル、メキシコ、アルゼンチン、その他のラテンアメリカ)、ヨーロッパ(英国、ドイツ、フランス、イタリア、スペイン、ハンガリー、ベルギー、オランダおよびルクセンブルグ、NORDIC(フィンランド、スウェーデン、ノルウェー) 、デンマーク)、アイルランド、スイス、オーストリア、ポーランド、トルコ、ロシア、その他のヨーロッパ)、ポーランド、トルコ、ロシア、その他のヨーロッパ)、アジア太平洋(中国、インド、日本、韓国、シンガポール、インドネシア、マレーシア) 、オーストラリア、ニュージーランド、その他のアジア太平洋地域)、中東およびアフリカ(イスラエル、GCC(サウジアラビア、UAE、バーレーン、クウェート、カタール、オマーン)、北アフリカ、南アフリカ、その他の中東およびアフリカ)