深部脳刺激装置市場は、予測期間中に約12.5%のCAGRを登録すると予想されています

深部脳刺激装置市場は、予測期間中に約12.5%のCAGRを登録すると予想されています。市場の成長を牽引する主な要因には、神経疾患のリスクと高い罹患率をもたらしている急速な高齢化、低侵襲処置の人気の高まりによるデバイスに対する需要の高まり、および技術的に高度な深部脳刺激デバイスの入手可能性が含まれます
- 深部脳刺激(DBS)は、進行性パーキンソン病(PD)、本態性振戦、およびジストニアに対して広く使用されている治療法である。しかし、過去30年間で、臨床応用の拡大に加えて、DBS技術には驚異的な成長がありました
- World Aginging 2019のレポートによると、2019年に世界では約7億300万人の65歳以上の人口があったと推定されています。この数字は、2050年に15億人に倍増すると予測されています。さらに、2019年には世界の6人に1人が65歳以上になるのは2050年までに、11人に1人の割合から増加しています。高齢者人口の数が増加すると予測されており、この人口は神経学的障害を起こしやすくなり、最終的には近い将来に市場を牽引する
- 現在、ヨーロッパと米国の規制当局によって承認された複数のDBSシステムがあります。米国食品医薬品局(FDA)は、ボストン・サイエンティフィック・コーポレーションのVercise Deep Brain Stimulation(DBS)システムを承認しました。このシステムは、パーキンソン病(PD)の症状を治療するために使用されます。さらに、研究者は既存のDBSシステムを強化しながら、次世代デバイスを開発しています.
研究者らはDBSを使用して脳のさまざまな領域をターゲットにし、パーキンソン病に罹患しているさまざまな集団での治療を研究しています。新しいシステムのいくつかの変更は、臨床転帰の改善を示している。それにもかかわらず、DBS技術におけるこれらの新しい発展は、大規模な患者コミュニティと医療提供者を奨励しています
したがって、DBS技術におけるこのような進歩はすべて、深部脳刺激装置市場の成長につながらなければならない
主な市場動向
パーキンソン病セグメントは、深部脳刺激装置市場で大きな市場シェアを保持すると予想されています
このセグメントの成長を牽引する主な要因は、パーキンソン病の世界的な有病率の上昇です。パーキンソン病財団2018によると、米国では約120万人が2030年までにパーキンソン病に罹患していると推定されています。これは、米国で2番目に多い神経変性疾患です。同様に、NHS UKの推定によると、パーキンソン病は500人に約1人が罹患していることが判明しており、これは英国で推定127000人がこの状態に苦しんでいることを意味します。さらに、検出されない何千人もの患者がいます。DBSは、パーキンソン病に対して最も一般的に行われる外科的治療である。これは通常、人々に行われます 少なくとも4年間パーキンソン病に罹患しており、薬の恩恵を受けているが、運動合併症を持っている人.さらに、DBSの分野では技術の進歩が高まっており、市場の全体的な成長を促進しています
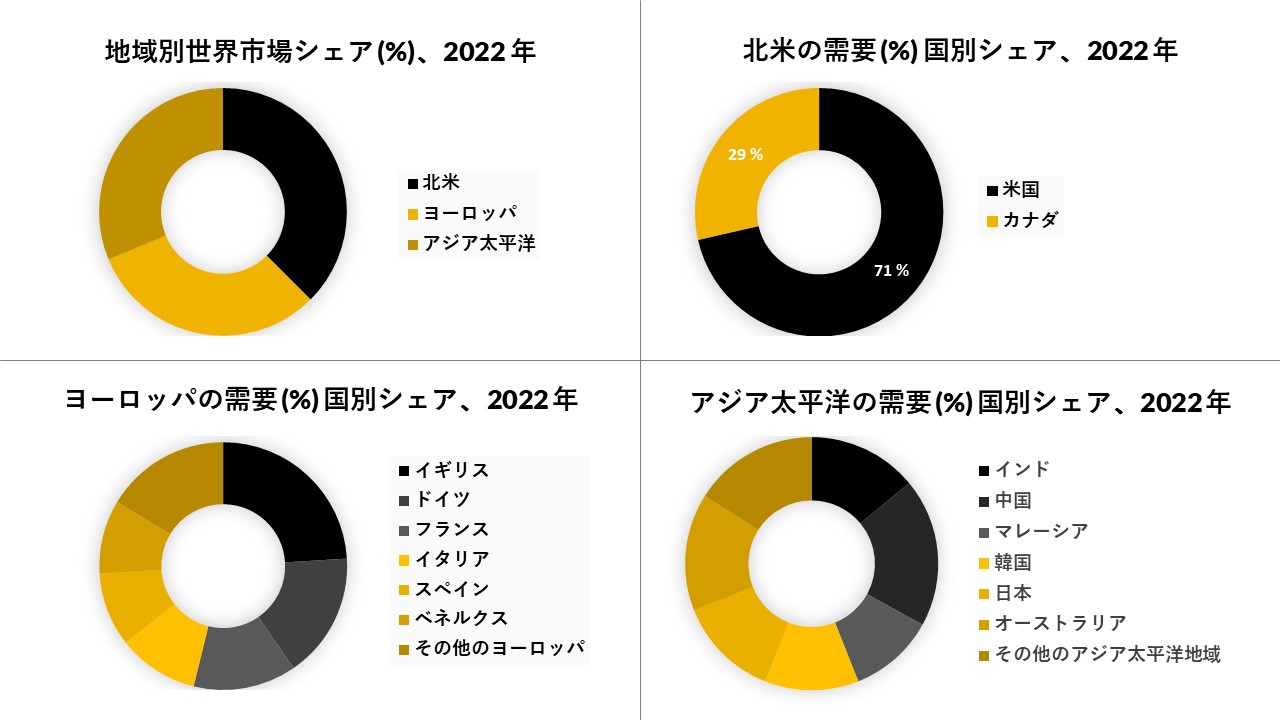
北米は市場で大きなシェアを占めると予想され、予測期間
パーキンソン財団の有病率プロジェクトによると、米国では2020年までに93万人がパーキンソン病に罹患していると推定されています。治療、障害、および同様の費用を含む米国におけるパーキンソン病の直接的および間接的な費用と、患者の労働不能による収入の損失の合計は、米国だけで年間250億米ドルと評価されると推定されています。したがって、医療システムに対するパーキンソン病の負担の増大は、米国の不確実な経済状況と相まって、相手先商標製品製造業者(OEM)に神経学的運動障害の治療のための費用対効果の高い装置を開発することを余儀なくされている
米国食品医薬品局(FDA)は、パーキンソン病と本態性振戦の治療のために、2015年に深部脳刺激(DBS)装置、すなわちBrio Systemを承認しました。Brio Systemの承認は、DBS技術のより迅速な開発を刺激する可能性があるため、患者コミュニティにとって朗報です
さらに、ボストン・サイエンティフィック・コーポレーションは、2017年12月に米国食品医薬品局(FDA)からVercise Deep Brain Stimulation(DBS)システムの承認を受けました。したがって、このようなすべての製品承認とパーキンソン病の発生率の増加は、深部脳刺激装置市場の成長を促進するのに役立っています.
競争環境
深部脳刺激装置市場は、いくつかの主要な市場プレーヤーの存在により、統合されています。市場プレーヤーには、アボット・ラボラトリーズ(セント・ジュード・メディカル)、北京ピンズ・メディカル、ボストン・サイエンティフィック・コーポレーション、フィッシャー・ウォレス、ファンクショナル・ニューロモジュレーション・リミテッド、メドトロニックPLC、ニューロペース・インク、レニショーPLC
このレポートを購入する理由:
- エクセル形式の市場予測(ME)シート
- 3ヶ月のアナリストサポート

北米(米国およびカナダ)、ラテンアメリカ(ブラジル、メキシコ、アルゼンチン、その他のラテンアメリカ)、ヨーロッパ(英国、ドイツ、フランス、イタリア、スペイン、ハンガリー、ベルギー、オランダおよびルクセンブルグ、NORDIC(フィンランド、スウェーデン、ノルウェー) 、デンマーク)、アイルランド、スイス、オーストリア、ポーランド、トルコ、ロシア、その他のヨーロッパ)、ポーランド、トルコ、ロシア、その他のヨーロッパ)、アジア太平洋(中国、インド、日本、韓国、シンガポール、インドネシア、マレーシア) 、オーストラリア、ニュージーランド、その他のアジア太平洋地域)、中東およびアフリカ(イスラエル、GCC(サウジアラビア、UAE、バーレーン、クウェート、カタール、オマーン)、北アフリカ、南アフリカ、その他の中東およびアフリカ)